What Type of Solid Does Ph3 Form
PH3 H - PH4. Rep gems come when your posts are rated by other community members.

Solved What Type Of Solid Will Each Of The Following Substances Form A Diamond E Kcl I Ar B Ph3 F Quartz J Cu C H2 G Nh4no3 K C6h12o6 D Mg H
The electronegativity of phosphorus and hydrogen is same so there is no formation of hydrogen bond from phosphine to water as phosphorus is larger in size and has same electronegativity as hydrogen.
. We review their content and use your feedback to keep the quality high. And carbon is one of the atoms that forms network solid. Classes of Crystalline Solids.
And so when it forms into a solid it will be a molecular solid. Acute short-term inhalation exposure to phosphine may cause headaches dizziness fatigue drowsiness burning substernal pain nausea vomiting cough labored breathing chest tightness pulmonary irritation pulmonary edema and tremors in humans. What type of solid will each of the following substances form.
The electronegativities of C and H are so close that C-H bonds are nonpolar. Experts are tested by Chegg as specialists in their subject area. The simplest type of substance is solid form but for ice it can be broken and reform to solid state.
What kind of solid is ca. PH3 also known as phosphine is insoluble in water as it is non polar and water is polar and like dissolves like so its insoluble in water. Which is the stronger acid PH3 or NH3.
PH3 is an acidNH3 is a base. There are four types of crystals. I dont get it it cant be covalent as theyre not sharing it cant be ionic as they dont have opposite charges it isnt metallic as they both arent metals so is it dative.
What type of intermolecular forces does CH4. What type of solid will each of the. Metals and ionic compounds typically form ordered crystalline solids.
Crystalline substances can be described by the types of particles in them and the types of chemical bonding that takes place between the particles. We know that diamond is made up of carbon atoms so that means that this will form an atomic solid. Report Thread starter 9 years ago.
The major intermolecular forces would be dipole-dipole forces and London dispersion forces. Three is just a a molecule. What is the bond formed and explain why.
Same thing with H two. 1 ionic 2 metallic 3 covalent network and 4 molecular. What solid does nh4no3 form.
Who are the experts. Substances that consist of large molecules or a mixture of molecules whose movements are more restricted often form amorphous solids. Properties and several examples of each type are listed in the.
There are no bond dipoles and no dipole-dipole interactions. So this will for made an atomic network solid. The entities of a solid phase may be arranged in a regular repeating pattern crystalline solids or randomly amorphous.
The only intermolecular forces in methane are London dispersion forces. 2 Terms wris190 Chem Ch 122-124 Identify the types of inter particle forces present in the solids of each of the following substances. Phosphine is used as an insecticide for the fumigation of grains animal feed and leaf-stored tobacco.
What type of solid will each of the following substances form. Ch 122-124 How does each of the following affect the rate of evaporation of a liquid in an open dish. Ammonium nitrate is the nitrate salt of the ammonium cation NH 4 NO 3 sometimes written as N 2 H 4 O 3 that is a white crystal solid and is highly soluble in water.
This problem has been solved. Select all that apply a PH3 atomic covalent network ionic metallic molecular b BaO atomic covalent network ionic metallic molecular c CaCO3 atomic covalent network ionic metallic molecular.
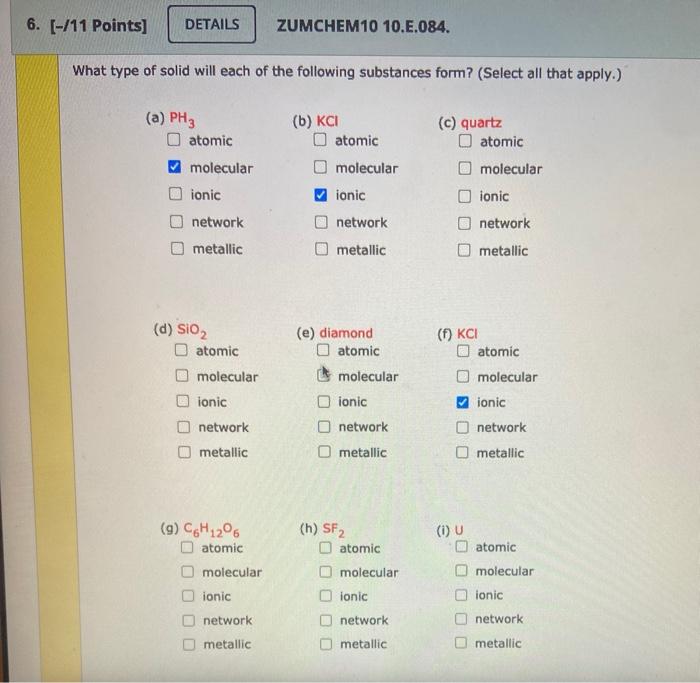
Solved 6 11 Points Details Zumchem10 10 E 084 What Chegg Com
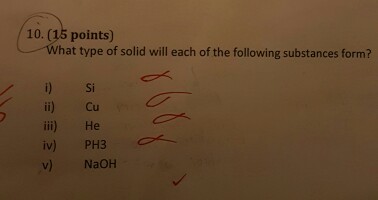
Solved What Type Of Solid Will Each Of The Following Chegg Com

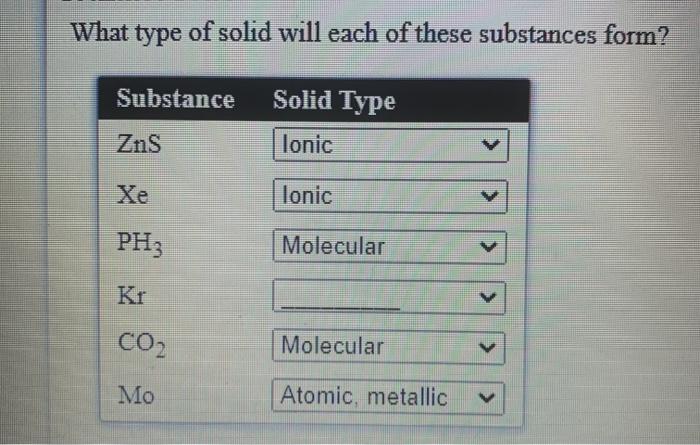
Solved What Type Of Solid Will Each Of These Substances Chegg Com

Solved 82 What Type Of Solid Will Each Of The Following Chegg Com
Comments
Post a Comment